Titanium is an element of the periodic table. Its symbol is Ti and its atomic number is 22. The metal has many properties like a very high level of corrosion resistance that make it an important element in many fields, for example in surgical and medical applications.

Image source: https://en.wikipedia.org/wiki/Titanium
What titanium is…

Discovered by William Gregor,1791
Image source: https://www.rsc.org/periodic-table/element/22/titanium
Titanium (Ti) is a chemical element of Group 4 (IVb) of the periodic table. It has a silvery-grey colouration. Titanium is Earth’s ninth most abundant element. It can be found in igneous rocks and their sediments. It is also present in plants, animals; natural waters, deep-sea dredgings; meteorites and stars. Titanium is a component of ilmenite, rutile, sphene and is present in titanates and many iron ores. The principal mined ores are located in Western Australia, Norway, Canada and Ukraine. Substantial deposits of rutile in North America and South Africa also contribute to the supply of titanium. World production of metals is approximately 90.000 tonnes per year, and titanium dioxide counts 4.3 million tonnes per year.
When is it first discovered?

Martin Heinrich Klaproth.
Image source: https://en.wikipedia.org/wiki/Titanium
Titanium was first identified in 1791 by the Reverend William Gregor. In 1795, Martin Heinrich Klaproth, a German scientist, examined a red mineral identified as Schörl from Hungary. It is a form of rutile (TiO2) and the scientist realised it was the oxide of a previously unknown element which he labelled as titanium. In 1910, Mattew A. Hunter, an employee of General Electric in the USA, made pure titanium metal by heating titanium tetrachloride and sodium metal.
Characteristics
- Pure titanium is ductile
- It can be polished to a high brilliance
- It has a very low electrical and thermal conductivity and is paramagnetic (weakly attracted to a magnet)
- It has excellent strength and corrosion resistance
- It is insoluble in water but soluble in concentrated acids
- The best solvents are hydrofluoric acid or other acids with fluoride ions.

Image source: https://www.britannica.com/technology/titanium-processing
Production

Image source: https://geology.com/minerals/ilmenite.shtml
Titanium(IV) oxide is produced on a commerce scale by either the “sulfate process” or the “chloride process”, both of which use the mineral ilmenite as a starting element. Pure titanium preparation is complex because of its reactivity. Titanium cannot be obtained by the common method of reducing the oxide with carbon. The element is pretty reactive to oxygen and nitrogen at high temperatures.
Applications
- Titanium has also been utilized as a deoxidizer in steel and as an alloying addition in many varieties of steels to degrade grain size, in stainless steel to reduce carbon content, in aluminium to purify grain size, and in copper to produce crystallisation.
- These alloys are employed in aircraft, spacecraft and missiles due to their low density and ability to resist extreme temperatures. They are also utilized in golf clubs, laptops, bicycles and crutches.
- Power plant condensers utilise titanium pipes because of their corrosion resistance, so the metal is used in desalination plants and to protect the hulls of ships, submarines and other structures exposed to seawater.
- Titanium attaches well to bones, so it is used in surgical applications such as joint replacements and tooth implants.
- Titanium(IV) oxide is the most used type of titanium on the market. It is extensively used as a pigment in paint, plastics, lacquers and paper. It is also a good infrared radiation reflector.
- Titanium(IV) oxide is used in sunscreens because it blocks UV light from reaching the skin.
- Pure titanium oxide is relatively clear, so it is used to produce titania, an artificial gemstone.
- Titanium tetrachloride (TiCl4), another compound, has been used to make smoke screens.
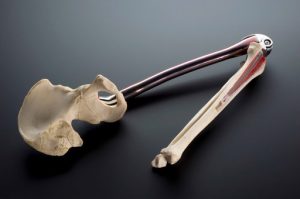
Image source: https://wellcomecollection.org/works/n766q9sy
Info sources: https://www.rsc.org/periodic-table/element/22/titanium
https://www.britannica.com/science/titanium
https://education.jlab.org/itselemental/ele022.html
https://www.lenntech.com/periodic/elements/ti.htm
https://www.chemicool.com/elements/titanium.html
